Supporting clinical study teams in engaging and retaining study participants as well as collecting real-time data remotely.
Digitising clinical trial participants' communication, data capture and retention
Clinical trials are complex to conduct. Study teams using digital clinical trial solutions can work more effectively and effortlessly to navigate and complete clinical trials and studies. A proper digital clinical trial captures all data without using paper forms during the study.
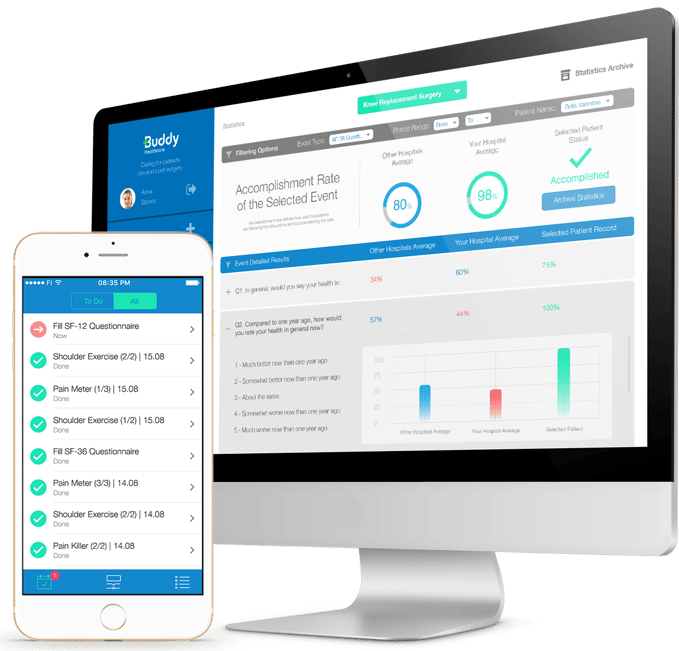
Buddy Healthcare's digital trial platform ensures digital data capture and engagement from study participants. The platform collects questionnaires such as PROMs/QoL, medication usage, or any other kind of forms, alongside provision to collect vital signs, such as blood pressure, body weight, pain scores, etc, all collected through a user-friendly VAS meter. Automated study participant communication and reminders enhance engagement, compliance and retention and help avoid missing data.
Buddy Healthcare’s digital solution enables sharing timed education and reminders with study participants, such as study-related instructions, study-site visit appointments or laboratory reminders, videos or essential links in an automated process.
The platform helps clinical trial professionals track and log trial-related tasks and notifies them of study participant deviations. The platform provides a simple and effortless view to the clinical trial teams, making participants' monitoring more manageable and efficient.
Buddy Healthcare's care coordination platform is a cost-effective and user-friendly digital tool for enhancing engagement, retention, automated data capture, and visualisation.
How does the digital clinical trial work?

Mobile application for study participants
- Transparent view of the protocol
- Automated reminders
- Enhanced retention and recruitment
- Easy remote completion of questionnaires

Dashboard for Study Personnel
- Collecting participants' data remotely
- Notifications when participants deviate from protocol
- Significantly reduces administrative work
- Pseudonymised and randomised data collection

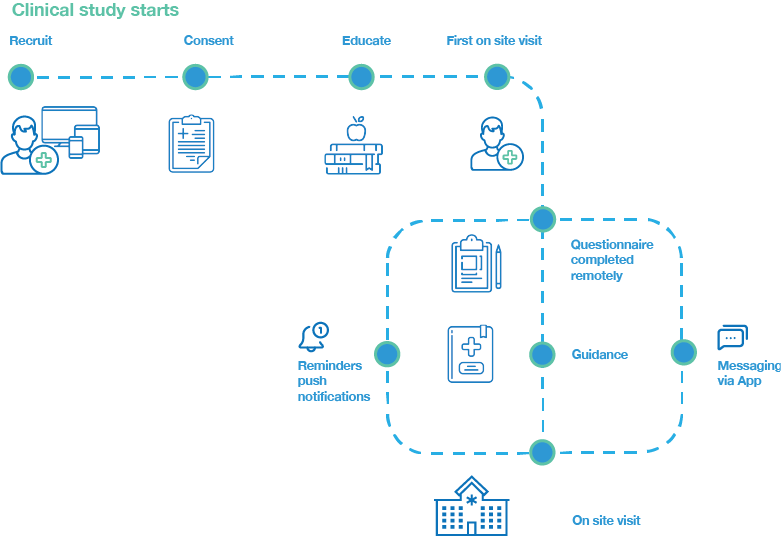
Clinical trial participant pathway
- example of a digital trial flow
How does Buddy Healthcare support clinical trials digitally?
(2).png?width=100&height=80&name=Untitled%20(100%20%C3%97%2080%20px)(2).png)
The participant's mobile application
- Transparent view of the protocol with timelines
- Automated reminders of important tasks
- Enhanced retention and recruitment
- Easy remote completion of questionnaires
(1).png?width=100&height=80&name=Untitled%20(100%20%C3%97%2080%20px)(1).png)
Automated data capture
- Automatically collects questionnaires and forms
- Pseudonymised and randomised data collection
- Data can also be collected by study personnel
- Collects the right information at the right time
.png?width=100&height=80&name=Untitled%20(100%20%C3%97%2080%20px).png)
Effortless participants' monitoring
- Tracks and visualises study participants’ progress
- Provides at a glance view of participants’ progress and notifies of deviations or missing data
- Automated reminders for participants of uncompleted tasks
Safe and Certified Platform
The Platform was co-developed with University Hospitals. Several University Hospitals, Regional Hospitals, Private Hospitals and clinical study teams use the platform safely.


